A sample gas has a volume of 10 L and a pressure of 1 ATM What is the new pressure if the volume is decreased to 3L? - Quora

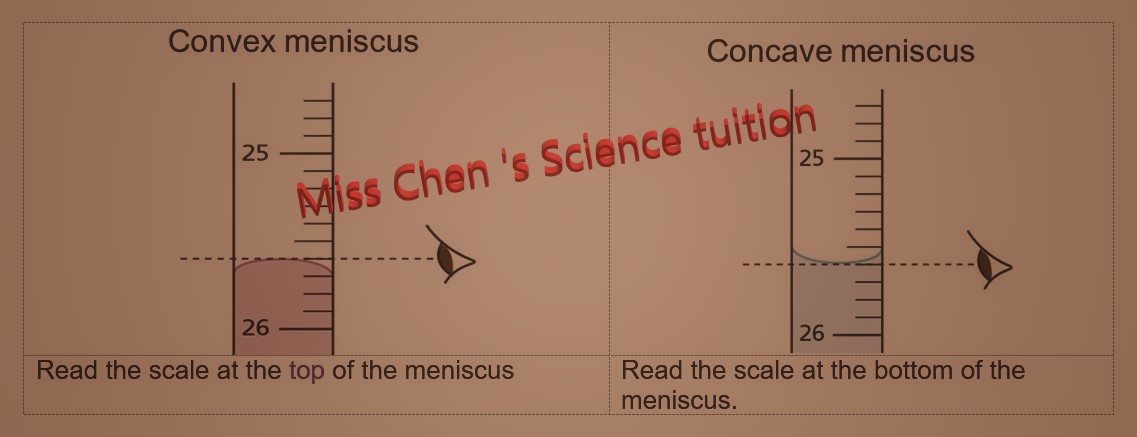
molar gas volume Avogadro's Law moles and mass calculations gcse chemistry calculations igcse KS4 science A level GCE AS A2 O Level practice questions exercises

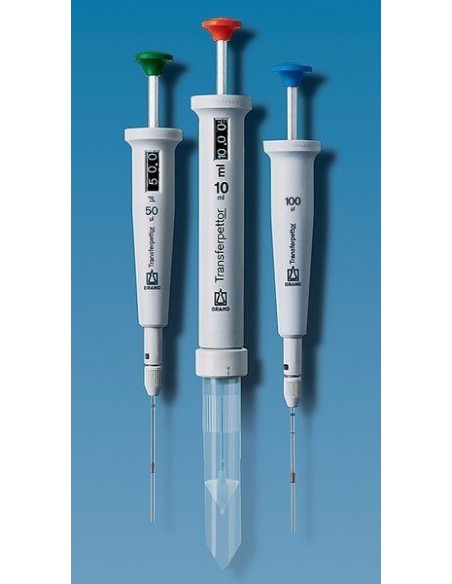
SOLVED: A syringe has a volume of 10.0 cm3 at pressure 1 atm. If you plug the end so that no gas can escape and push the plunger down, what must be
A syringe has a volume of 10.0 cm^3 at pressure 1 atm. If you plug the end so that no gas can escape and push the plunger down, - Sarthaks eConnect

VIVEX: A Formula for Calculating Individual Vitreous Volume: A New Approach Towards Tailored Patient Dosing Regime in Intravitreal Therapy | Ophthalmology and Therapy

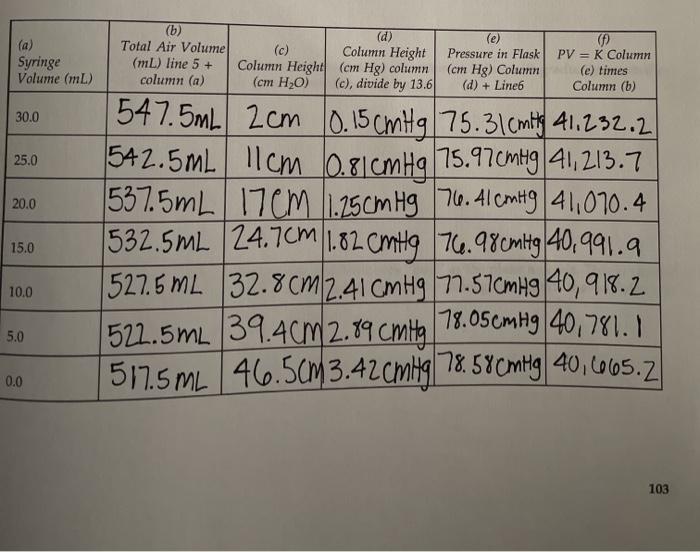
PDF) A System for Real-Time Syringe Classification and Volume Measurement Using a Combination of Image Processing and Artificial Neural Networks
A syringe contains a gas. The plunger was released so the gas inside could expand. Assuming the increase in volume was 134 mL, what is the amount of work done (in J)? -

SOLVED: A syringe has a volume of 10.0 cm3 at pressure 1 atm. If you plug the end so that no gas can escape and push the plunger down, what must be

A syringe has a volume of `10.0 cm^3` at pressure 1 atm. If you plug the end so that no gas can escape and push the plunger down, what be the final volume to change the pressure to 3.5 atm?

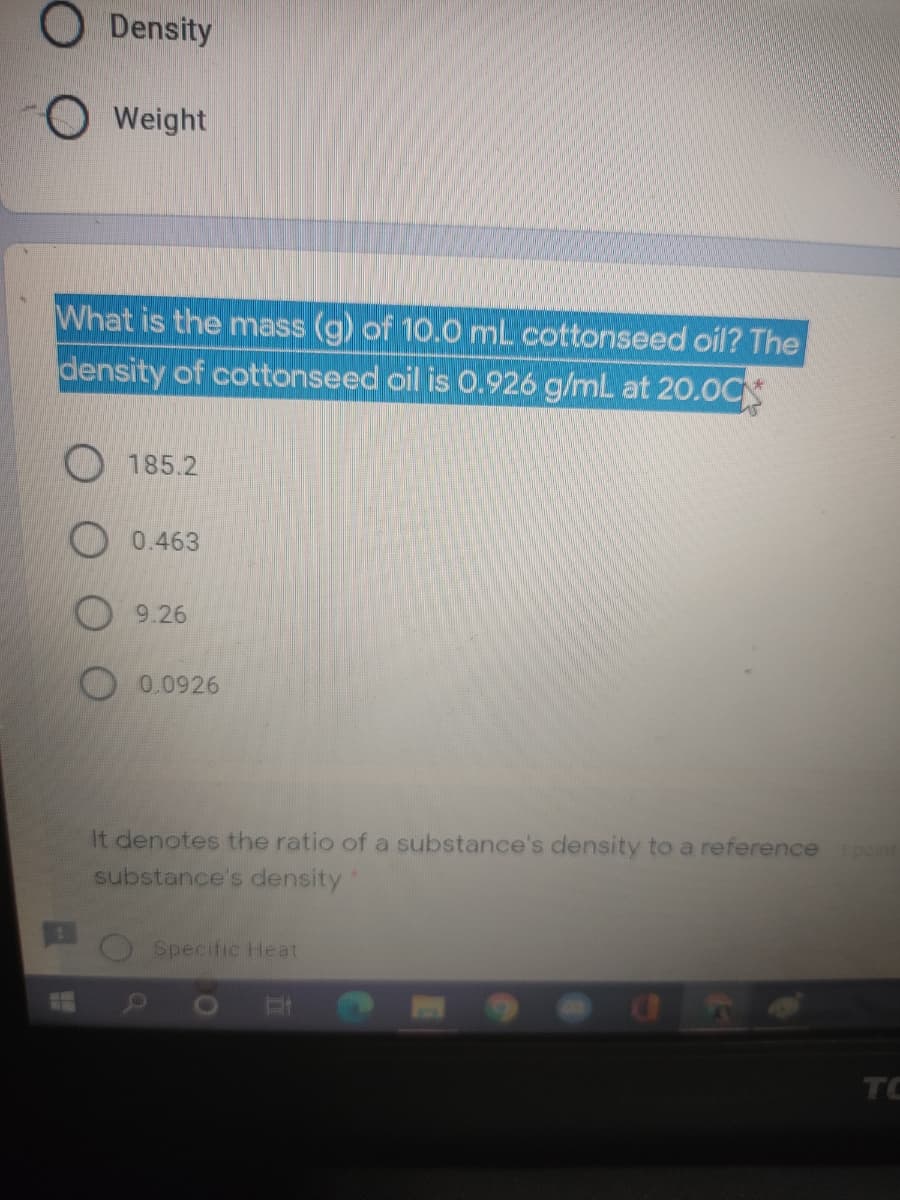
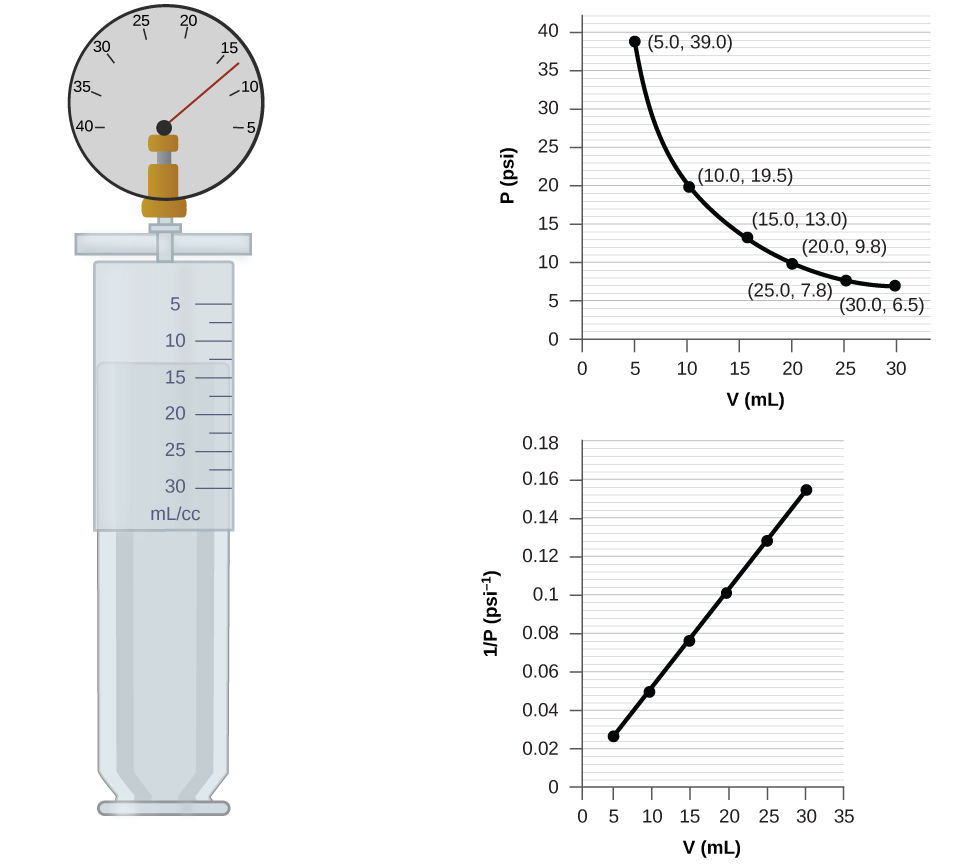
8.2: Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law | General College Chemistry I





![Marathi] A syringe has a volume of 10.0 cm^3 at pressure 1 atm. If yo Marathi] A syringe has a volume of 10.0 cm^3 at pressure 1 atm. If yo](https://static.doubtnut.com/ss/web/11274255.webp)